Future-Ready Cancer Screening: What Every Clinician Should Know in 2025
OncologyPage Navigation
The State of Cancer Screening in 2025
Cancer screening in 2025 has entered a new era, marked by technological sophistication, precision approaches, and evolving global standards. Traditional tools like mammography, colonoscopy, and Pap smears remain in use, but they’re increasingly complemented and in some cases replaced by molecular and AI-driven methods. Tools like multi-cancer early detection (MCED) blood tests, liquid biopsies, and low-dose CT scans are redefining what early detection means across a spectrum of cancers.
Governments and healthcare systems worldwide are prioritizing population-level screening programs, particularly in high-burden cancers such as breast, colorectal, cervical, prostate, and lung. However, the emphasis has shifted from one-size-fits-all protocols to risk-adapted strategies, which consider genetics, lifestyle, and demographic factors.
In addition, digital health tools including AI-enabled triage systems and patient engagement apps have improved participation and follow-up compliance. Clinicians must now be proficient not just in ordering tests, but in interpreting results across imaging, histology, and molecular diagnostics.
Globally, the World Health Organization (WHO) and regional cancer agencies continue to push for universal screening access, particularly in underserved regions. For the modern clinician, staying updated with screening innovations, guidelines, and disparities is critical to delivering equitable and effective cancer prevention and care.
Advancing Knowledge with Cancer Screening CME Online

Continuing Medical Education (CME) in cancer screening is essential in 2025, where rapid advancements in diagnostic tools and evolving guidelines demand ongoing professional development. Online CME platforms have revolutionized how clinicians learn providing flexible, accredited, and clinically relevant education from anywhere, at any time.
Top platforms like ASCO University, Medscape CME, NCCN Learning Portal, and UptoDate offer focused modules on cancer screening, covering traditional methods as well as emerging technologies like liquid biopsy, MCED, and AI-enhanced imaging. These courses often include interactive case discussions, clinical vignettes, and knowledge checks to reinforce retention.
Specialty-focused CME (e.g., for primary care, OB-GYN, radiology, oncology) ensures clinicians can tailor learning to their practice needs. Many platforms also track CME credits automatically and integrate with Maintenance of Certification (MOC) systems.
Online CME also promotes lifelong learning, helping clinicians adapt to new screening recommendations issued by bodies like the USPSTF, ESMO, and ACS. Beyond technical knowledge, modules often explore topics like shared decision-making, communication of risk, and addressing screening disparities.
With cancer screening becoming more personalized and tech-driven, CME ensures clinicians remain confident, current, and compliant in offering the best possible preventive care to their patients.
Board Preparation and Certification Pathways

Certification in cancer screening reflects a clinician’s expertise in early detection, guideline adherence, and diagnostic proficiency. In 2025, board preparation pathways vary based on specialty but are increasingly aligned with evolving standards in screening science, genomics, and technology-assisted detection.
For primary care physicians, preventive medicine boards and internal medicine subspecialty certifications emphasize population screening strategies, risk stratification, and evidence-based decision-making. Radiologists preparing through the American Board of Radiology (ABR) must master breast imaging, lung nodule assessment, and interpretation of screening modalities like low-dose CT and digital mammography.
Pathologists focus on screening specimen evaluation and biomarker-based interpretation, often preparing through the American Board of Pathology (ABPath). Oncologists encounter cancer screening content as part of ABIM oncology boards, especially in understanding how screening findings guide early intervention.
Certification review typically includes MCQs, case vignettes, image interpretation, and clinical algorithms. Many clinicians use platforms like BoardVitals, UWorld, and TrueLearn, which reflect current screening guidelines and new diagnostic technologies.
In 2025, achieving certification not only boosts professional credibility but also ensures that clinicians are up to date with evolving protocols delivering better patient outcomes through timely, appropriate cancer screening and prevention strategies.
In 2025, fellowship programs focused on cancer screening are shaping the next generation of leaders in early detection and preventive oncology. These programs offer specialized training in the integration of screening technologies, risk assessment models, population health strategies, and diagnostic innovation. Fellowships are available across disciplines, including oncology, radiology, pathology, public health, and molecular medicine.
Notable institutions such as MD Anderson Cancer Center, Memorial Sloan Kettering, and Johns Hopkins offer advanced programs in oncologic imaging, screening program design, and cancer prevention research. Many fellowships now include rotations in genomic laboratories, community screening outreach, and AI-based diagnostic platforms.
Trainees engage in clinical duties, research projects, and often policy-making activities equipping them to lead initiatives such as expanding access to screening, designing mobile diagnostic units, or implementing screening algorithms in diverse populations. There is also increasing focus on health equity, training fellows to address disparities in early detection through culturally informed outreach and screening delivery models.
These fellowships bridge academia, technology, and clinical care. By the end of training, fellows are not only experts in screening modalities but also innovators capable of transforming how and where cancer is detected, ultimately improving population-level cancer outcomes.
Cancer Screening Education for Medical Students
Integrating cancer screening into undergraduate medical education is a priority in 2025. With early detection linked directly to improved survival, today’s medical students must graduate with a clear understanding of screening guidelines, risk factors, diagnostic tools, and patient communication techniques.
Curricula now include focused modules on breast, colorectal, cervical, lung, and prostate cancer screening, along with newer topics like MCED tests, liquid biopsies, and genetic screening. Case-based learning is commonly used, allowing students to simulate real-world scenarios such as identifying candidates for screening, ordering appropriate tests, and navigating false positives.
In addition, clinical rotations in oncology, radiology, and primary care expose students to screening implementation and shared decision-making. Some schools offer electives or research opportunities in cancer prevention, encouraging students to explore innovation and policy in early detection.
Medical schools are also training students to use digital tools such as AI-assisted imaging software and patient risk calculators. Ethical aspects of screening such as overdiagnosis, informed consent, and access equity are emphasized through interdisciplinary discussions.
By embedding screening education early, future clinicians are better prepared to advocate for preventive care, personalize screening strategies, and contribute meaningfully to reducing the cancer burden in their communities.
Free Learning Resources for Cancer Screening Professionals

In 2025, a wide array of high-quality, free learning resources empower clinicians to stay current with cancer screening innovations and best practices. These tools are especially valuable for professionals in training, rural providers, and global health practitioners seeking access to the latest evidence without financial barriers.
Leading sources include open-access journals such as BMC Cancer, Cancer Medicine, and JNCI Cancer Spectrum, which publish peer-reviewed articles on screening protocols, biomarkers, and epidemiology. PubMed Central and Google Scholar offer searchable access to thousands of free publications.
Professional societies like the American Cancer Society (ACS), National Cancer Institute (NCI), and European Society for Medical Oncology (ESMO) host free webinars, guideline updates, and downloadable screening algorithms. The World Health Organization (WHO) provides multilingual toolkits designed for global implementation of cancer screening programs.
YouTube channels from academic hospitals and oncology educators feature expert-led videos, diagnostic walkthroughs, and case reviews. Mobile apps such as NCCN Guidelines, Cancer.Net, and ASCO's Cancer Education App deliver real-time guidance at the point of care.
These resources support lifelong learning and democratize knowledge, ensuring that clinicians regardless of location or budget can confidently apply the most up-to-date cancer screening practices in patient care.
Review Courses for Clinicians: What to Look For
In 2025, review courses are a cornerstone for clinicians seeking to refresh their cancer screening knowledge, prepare for certification exams, or keep up with evolving best practices. With the growing complexity of screening guidelines and diagnostic options, an effective review course must go beyond static slide decks and offer interactive, evidence-based, and specialty-relevant content.
Top-tier review programs such as those from Harvard CME, Mayo Clinic, ASCO, and BoardVitals feature comprehensive modules covering cancer-specific screening tools (e.g., mammography, colonoscopy, LDCT), genomic and biomarker screening, and newer innovations like liquid biopsy and MCED. They also emphasize case-based learning, diagnostic flowcharts, and real-world clinical scenarios.
When choosing a course, clinicians should look for features like live Q&A sessions, mock exam questions, CME/MOC credit availability, and frequent guideline updates. Courses tailored by role primary care vs. oncology vs. radiology ensure that learning is practice-relevant.
Some platforms now offer adaptive learning using AI to personalize content and reinforce weak areas. This approach enhances retention and exam performance.
Ultimately, a high-quality review course should not only prepare clinicians for assessments but also strengthen their clinical judgment and diagnostic confidence in real-time patient care.
Latest Research Driving Change in Screening Protocols
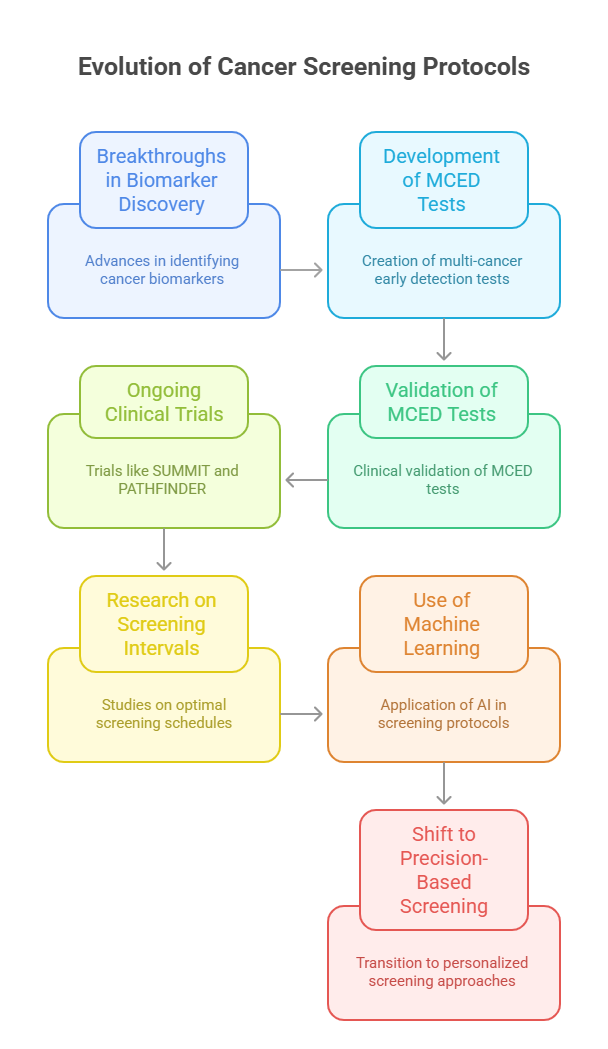
Cutting-edge research in 2025 is reshaping cancer screening protocols, enabling earlier detection, improved specificity, and broader reach across populations. This transformation is driven by breakthroughs in biomarker discovery, molecular diagnostics, and real-world clinical data.
Recent publications have validated the clinical utility of multi-cancer early detection (MCED) tests, which use blood-based analysis of circulating tumor DNA (ctDNA) and methylation patterns to detect dozens of cancers from a single sample. Studies in the New England Journal of Medicine and The Lancet Oncology report that MCED tests demonstrate high specificity with minimal false positives, prompting major policy discussions on implementation.
Ongoing trials like SUMMIT, PATHFINDER, and STRIVE continue to generate evidence on screening efficacy, patient acceptance, and cost-effectiveness. Other notable studies have explored low-dose CT for lung cancer, MRI screening in high-risk breast cancer patients, and HPV DNA testing for cervical cancer.
Importantly, research is focusing not only on the test itself, but on optimal screening intervals, age thresholds, and risk-adapted models. Machine learning and population modeling are being used to update clinical guidelines dynamically based on real-time data.
This body of evidence is moving screening from a one-size-fits-all model to a precision-based, dynamic approach, grounded in the latest science.
Emerging Clinical Trials in Cancer Screening
Clinical trials in 2025 are at the forefront of transforming cancer screening into a more precise, accessible, and impactful process. These trials aim to validate new technologies, evaluate screening in previously unaddressed populations, and refine guidelines for early detection.
One of the most anticipated areas of research is multi-cancer early detection (MCED). Trials like PATHFINDER 2, NHS-Galleri, and SPOTLIGHT are testing MCED tools across thousands of asymptomatic participants to assess cancer detection rates, diagnostic follow-up efficiency, and long-term survival outcomes. These studies could potentially pave the way for blood-based screening to become a standard component of preventive care.
Other trials are exploring AI-augmented imaging interpretation for mammograms, chest CT scans, and colonoscopies. By reducing human error and inter-reader variability, these studies hope to improve diagnostic yield without increasing resource burden.
Additionally, community-based screening trials are targeting underserved populations to evaluate the feasibility of mobile units, self-collection kits, and telehealth-supported screening initiatives.
Clinicians are encouraged to stay informed about ongoing trials through platforms like ClinicalTrials.gov, NIH Cancer Trials, and ESMO’s trial repository. Participating in or referring eligible patients to these studies not only enhances care quality but also helps accelerate the adoption of the next generation of life-saving screening tools.
Digital Tools Enhancing Screening Accuracy and Workflow
In 2025, digital tools are integral to improving cancer screening workflows, enhancing diagnostic accuracy, and enabling timely interventions. These tools range from AI-powered diagnostic platforms to mobile apps and cloud-based decision support systems, all designed to streamline clinical processes and minimize errors.
Artificial intelligence (AI) has become especially valuable in screening programs. In radiology, AI algorithms interpret mammograms, chest CTs, and colonoscopy videos flagging subtle abnormalities that may escape the human eye. In pathology, AI aids in histological slide analysis, allowing for rapid and standardized diagnoses.
Clinical decision support systems (CDSS) are now embedded in electronic health records (EHRs), prompting clinicians when patients meet criteria for age-based or risk-based cancer screening. These alerts help ensure guideline adherence and prevent missed opportunities for early detection.
Patient-facing mobile applications enhance engagement by offering personalized screening schedules, test result tracking, and educational modules. Apps also send automated reminders, improving adherence and reducing no-show rates.
Digital platforms support tele-screening in rural and underserved areas, allowing remote triage and interpretation of results. As digital tools continue to evolve, they promise to create a more connected, efficient, and equitable screening ecosystem; empowering both clinicians and patients in the fight against cancer.
Genomic and Biomarker-Based Screening Innovations

The field of cancer screening has taken a quantum leap in 2025, thanks to advancements in genomic profiling and biomarker discovery. These innovations are moving screening beyond anatomical imaging and into the realm of molecular-level detection, enabling earlier and more precise identification of malignancies.
One of the most promising developments is the use of circulating tumor DNA (ctDNA) and epigenetic signatures in blood to detect cancer presence; often before symptoms arise. Multi-cancer early detection (MCED) blood tests now utilize genomic sequencing and methylation analysis to pinpoint the tissue of origin, offering a revolutionary approach to early detection.
Other emerging biomarkers include microRNAs, exosomes, protein expression profiles, and tumor-associated autoantibodies. These are being explored in cancers that historically lacked reliable screening tools, such as pancreatic, ovarian, and gastric cancers.
Genomic screening is also being used to stratify risk. For instance, individuals with BRCA, APC, or Lynch syndrome mutations may begin screening earlier or with different modalities. Precision screening enables clinicians to tailor protocols to genetic predisposition, reducing over-screening and under-detection.
These breakthroughs are turning cancer screening into a personalized science; one where molecular fingerprints guide both detection and prevention strategies, optimizing outcomes and resource use.
Post-Screening: Therapy and Treatment Pathways
The value of cancer screening doesn’t end with detection; it’s what follows that defines patient outcomes. In 2025, post-screening pathways are highly structured, integrating diagnostic precision with coordinated treatment planning to ensure timely and effective care.
When a screening test returns positive, the next steps involve confirmatory diagnostics such as advanced imaging, tissue biopsy, and molecular profiling. Speed is critical; many health systems now operate under diagnosis-to-treatment benchmarks, ensuring patients enter treatment pathways without delay.
Multidisciplinary tumor boards assess each case, bringing together oncologists, radiologists, pathologists, and surgeons to review results and determine the optimal approach. Treatment may include surgery, radiation, chemotherapy, immunotherapy, or targeted therapy, depending on the cancer’s type, stage, and biomarker profile.
For cancers detected at early stages thanks to modern screening methods treatment is often less invasive and more successful. In contrast, ambiguous or false-positive results require careful communication to prevent overtreatment and anxiety.
Clinicians must also support patients in navigating the transition from screening to therapy, addressing concerns, expectations, and psychological impacts. In 2025, post-screening care is not a siloed process but a continuum of coordinated, patient-centered oncology, grounded in diagnostic precision and compassionate practice.
Addressing Disparities in Screening Access
Despite major advances in cancer screening, disparities in access remain a pressing concern in 2025. Factors such as socioeconomic status, geographic location, race/ethnicity, health literacy, and insurance coverage continue to influence who gets screened and when.
Rural populations often face logistical barriers like transportation, limited specialist availability, or lack of diagnostic equipment. Urban underserved communities may encounter institutional mistrust, low awareness, or cost-related obstacles. Additionally, language barriers and cultural misconceptions can hinder participation among immigrant and minority populations.
To close these gaps, health systems are investing in community-based screening programs, mobile diagnostic units, and self-collection kits for cervical, colorectal, and now even blood-based cancer screening. These methods are proving especially effective in reaching under-screened groups.
Digital outreach via text messages, multilingual apps, and telehealth counseling has improved screening education and follow-up rates. Some states and nations have implemented navigation programs, where community health workers guide patients through screening and care coordination.
Moreover, equity-focused policies from organizations like the American Cancer Society, NIH, and World Health Organization are setting benchmarks for universal access. In 2025, addressing disparities is not a side goal, it's a core priority for clinicians, administrators, and policymakers committed to equitable early cancer detection.
Ethical and Communication Challenges in Screening
Cancer screening, while lifesaving, introduces complex ethical and communication challenges that clinicians must navigate thoughtfully in 2025. These include issues around false positives, overdiagnosis, incidental findings, informed consent, and psychological distress.
False-positive results, especially in mammography and low-dose CT scans, can lead to unnecessary biopsies and emotional trauma. Conversely, false negatives may create false reassurance. Overdiagnosis detecting cancers that would never become clinically significant can result in overtreatment and long-term morbidity. Clinicians must weigh benefits and harms when recommending screening, particularly in older adults or those with comorbidities.
Informed consent is paramount. Patients need clear, balanced information about test sensitivity, specificity, follow-up procedures, and potential outcomes. In 2025, decision aids and visual tools help clinicians explain complex data in understandable terms.
Communication after a positive result is equally crucial. Delivering bad news requires empathy, clarity, and support. Collaboration with psychologists or patient navigators can ease this burden.
Ethical dilemmas also arise when screening uncovers non-cancer findings, such as aneurysms or unrelated genetic mutations. Clear protocols for disclosure and follow-up are essential.
Ultimately, ethical screening is not just about technology it’s about trust, transparency, and shared decision-making, ensuring that patients are active, informed participants in their care.
The Future of Cancer Screening: Toward Personalized Prevention
Cancer screening in 2025 is no longer a one-size-fits-all process; it is rapidly evolving into a model of personalized prevention, where risk, biology, and behavior dictate who gets screened, when, and how. This future-forward approach leverages genomics, AI, lifestyle data, and real-world evidence to tailor strategies for individual patients.
Risk calculators using inputs like family history, genomic data, and social determinants of health generate personalized screening schedules. For instance, a woman with BRCA1 mutations may begin breast cancer screening with MRI in her 20s, while an average-risk patient starts mammography at 40 or later. Men with high polygenic risk scores for prostate cancer may undergo earlier or more frequent PSA monitoring.
Digital health tools now integrate screening data with lifestyle metrics from wearables (e.g., physical activity, sleep, smoking status), creating dynamic prevention plans that evolve with the patient’s profile. AI models continuously update risk and suggest appropriate interventions.
Looking ahead, researchers are exploring cancer vaccines, immune-based interception, and targeted chemoprevention in high-risk populations. Population health strategies are also becoming more proactive, with national databases driving early outreach and education.
In essence, the future of screening lies not in just finding cancer early, but in preventing it intelligently before it ever begins
Read more such content on @ Hidoc Dr | Medical Learning App for Doctors
Recommended News For You
Recommended Articles For You
Featured News
Featured Articles
Featured Events
Featured KOL Videos
1.
Le cancer et le COVID ont conduit le patient à une double transplantation de poumon.
2.
Effective for localizing small, non-palpable breast lesions is ultrasound-guided localization with magnetic seeds.
3.
Long-term study links chronic conditions in midlife to higher cancer risk and mortality
4.
Subcutaneous Cancer Immunotherapies Provide New Options for Physicians and Patients
5.
When does a melanoma metastasize? Implications for management
1.
Unlocking the Mysteries of Reticulocyte Counts: A Guide to Understanding Your Blood Results
2.
The Checkpoint Architect: Unraveling the Mechanisms of PD-L1 Regulation for the Next Generation of Small-Molecule Therapies
3.
Screening Efficacy, Molecular Precision, and Therapeutic Revolutions in Lung Cancer 2025
4.
Genetic Testing in Cancer Prevention: BRCA Mutations and Lynch Syndrome Unlocked
5.
Transforming Cancer Care: CAR T-Cell Therapy for Relapsed/Refractory NHL and ALL
1.
International Lung Cancer Congress®
2.
Genito-Urinary Oncology Summit 2026
3.
Future NRG Oncology Meeting
4.
ISMB 2026 (Intelligent Systems for Molecular Biology)
5.
Annual International Congress on the Future of Breast Cancer East
1.
Revolutionizing Treatment of ALK Rearranged NSCLC with Lorlatinib - Part II
2.
Management of 1st line ALK+ mNSCLC (CROWN TRIAL Update)
3.
An In-Depth Look At The Signs And Symptoms Of Lymphoma
4.
Post Progression Approaches After First-line Third-Generaion ALK Inhibitors
5.
Pazopanib: A Game-Changer in Managing Advanced Renal Cell Carcinoma - Part IV
© Copyright 2025 Hidoc Dr. Inc.
Terms & Conditions - LLP | Inc. | Privacy Policy - LLP | Inc. | Account Deactivation

